By Sola Ogundipe
A 28-year-old man, Oscar Murphy, has become the first patient in the UK to receive a revolutionary “living drug” on the NHS for his specific form of blood cancer.
Murphy, who is battling B-cell Acute Lymphoblastic Leukemia (B-cell ALL), recently underwent a pioneering procedure known as CAR-T therapy with the drug obe-cel, represents a radical shift in oncology by moving away from traditional mass-produced chemicals and toward a highly personalised, genetic approach to medicine.
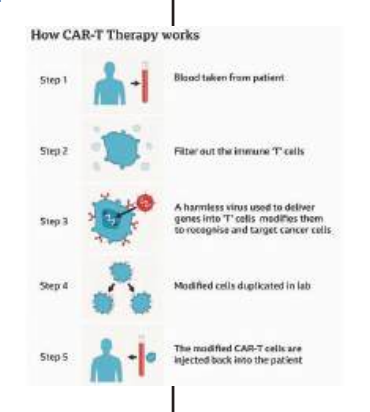
The process of creating the “living medicine” described as a marvel of modern bioengineering, begins with clinicians harvesting a patient’s own immune cells, or T-cells, which are then sent to a specialised laboratory where the cells are genetically reprogrammed to recognise and target a specific protein found on the surface of cancer cells.
The super-charged cells are returned to the patient’s body through an intravenous drip, and they function as a biological surveillance system, multiplying and hunting down the leukemia with a level of precision far beyond traditional treatments.
For Murphy, the therapy offers a vital lifeline against B-cell ALL, an aggressive cancer where the bone marrow produces an overabundance of immature B-cells, known as blasts. The dysfunctional cells eventually crowd out healthy blood cells, leaving patients vulnerable to frequent infections, chronic fatigue, and easy bruising.
While this form of leukemia is the most common type diagnosed in children, it has historically been much more difficult to manage in adults. The approval of obe-cel for adult use on the NHS marks a significant expansion of care, providing a new option for those who have exhausted conventional therapies.
The procedure took place at the Manchester Royal Infirmary, one of the specialist centers selected by NHS England to deliver this complex treatment. It requires eligible patients to receive two doses exactly 10 days apart. Murphy received his initial dose on January 2nd and his final dose the following Monday, officially transitioning the treatment from the realm of clinical trials into routine specialised care, leveraging the patient’s own biology to fight the disease.
It is estimated that around 50 patients will benefit from the treatment every year, which has been described as ‘hope for a cure’.
In a clinical trial, 77 per cent of patients went into remission after treatment, with half showing no signs of cancer after three and a half years. On average, the treatment gave patients 15.6 additional months of life.
In the view of NHS National Clinical Director for Cancer, Professor Peter Johnson, “This cutting-edge therapy has shown real promise in trials and could give patients with this aggressive form of leukaemia a chance to live free from cancer for longer – and, for some, it could offer the hope of a cure.”
Murphy was diagnosed with B-cell ALL in March 2025, and underwent chemotherapy and a donor stem cell transplant in July.
But in November he was told that his cancer had returned. “The leukaemia I’ve got is so fast-acting. It needs an even quicker response to stop it, and we’ve now got an answer for that.
“It’s very sci-fi, but if it means it gets rid of the cancer permanently and my own cells can do it it’s just fantastic,” he said.
The treatment will be offered to people aged 26 and over living with B-cell ALL which has returned or not responded to previous treatment, following approval from the National Institute for Health and Care Excellence.
Murphy’s haematologist, Dr Eleni Tholouli, said: “Usually, this type of leukaemia is very aggressive and adult patients don’t live beyond six to eight months. With this therapy, we are able to offer them years and potentially a cure. It’s very significant and is revolutionising the way we tackle this cancer.”
The post First adult patient receives genetically modified ‘living drug’ for leukemia appeared first on Vanguard News.